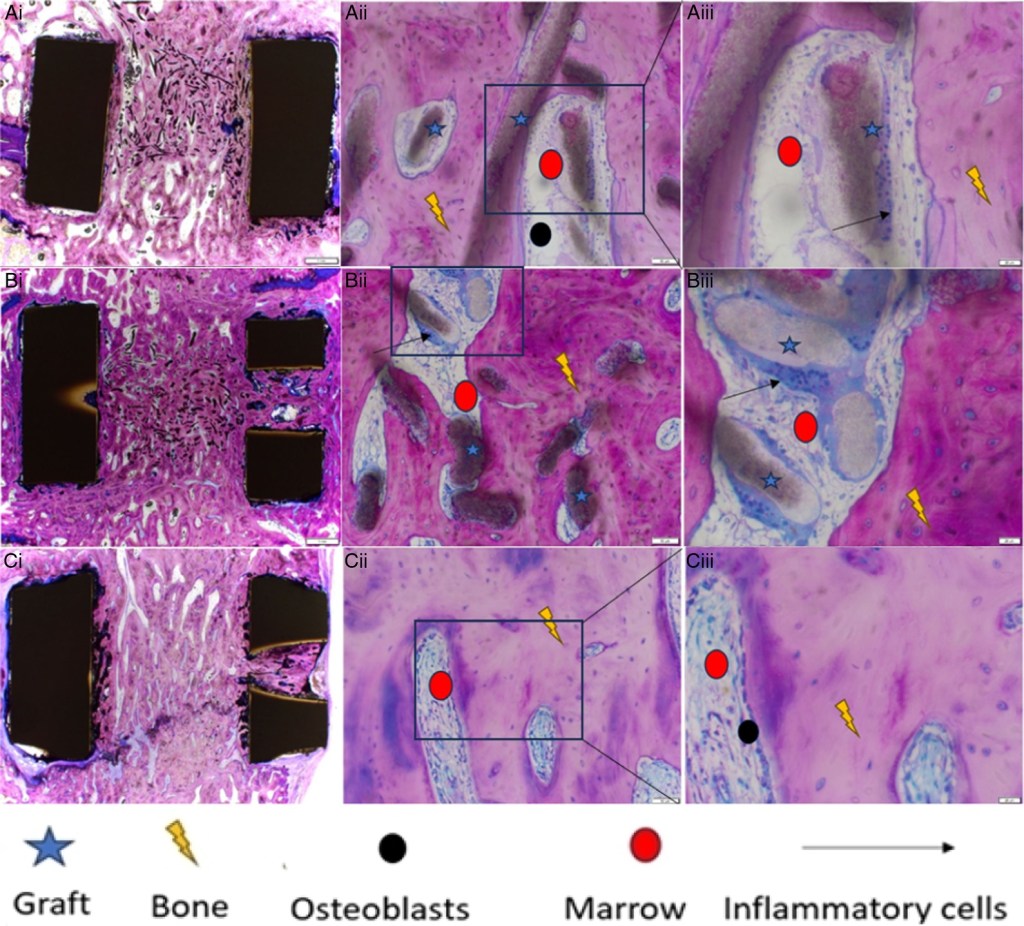
Theradaptive announced the publication of a peer-reviewed preclinical study in Spine that highlights the safety and efficacy of OsteoAdapt™ SP, a therapeutic candidate for spinal fusion. In a study on sheep, OsteoAdapt SP, which combines AMP2—a modified bone-forming protein—with a synthetic bone graft, demonstrated faster and stronger bone formation compared to the standard autologous iliac crest bone graft (ICBG). The study observed outcomes at 8, 16, and 26 weeks, showing promising results in bone quality, mineralization, and fusion across different cage materials (PEEK and Titanium) and dosages. Currently, OsteoAdapt SP is undergoing Phase I/II clinical trials (OASIS) for treating degenerative disc disease in humans, aiming to reduce the need for traditional autografts in spinal surgeries.[1]
Click for Full Journal Article in Spine
For background information, check out my former post at: Theradaptive Receives 2024 Best Technology in Spine Award by Orthopedics This Week
References
[1] Theradaptive. (2024c, November 6). Theradaptive Announces Peer-Reviewed Publication in Spine of Preclinical Spinal Fusion Study of OsteoAdaptTM SP. PR Newswire. https://www.prnewswire.com/news-releases/theradaptive-announces-peer-reviewed-publication-in-spine-of-preclinical-spinal-fusion-study-of-osteoadapt-sp-302296711.html
Image: Figure 6, high-magnification histology at 26 weeks post-implantation https://journals.lww.com/spinejournal/pages/imagegallery.aspx?year=2024&issue=10010&article=00011
Leave a comment