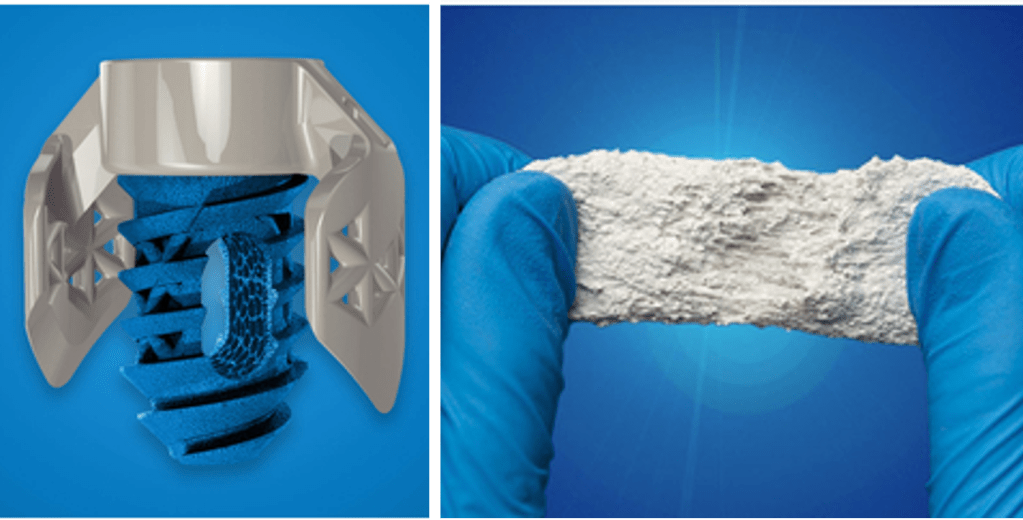
SurGenTec LLC has won two 2024 Spine Technology Awards for their innovative medical devices. The TiLink-P Posterior Sacroiliac (SI) Joint Fusion System received the Gold Award for its unique approach to treating chronic SI joint pain. The OsteoFlo HydroFiber, a synthetic bone graft, won the Bronze Award. Both products are designed to improve patient outcomes in spine surgery. SurGenTec is committed to developing cutting-edge technologies that enhance physician capabilities and deliver exceptional patient care.[1]
“We are truly honored to receive these prestigious awards for our innovative spine surgery products. The Spine Technology Awards are highly competitive, and securing two in a single year is a remarkable achievement. At SurGenTec, our mission is to pioneer cutting-edge technologies that enable physicians to deliver exceptional patient care. These accolades reflect our team’s unwavering commitment to advancing the field of spine surgery and pushing the boundaries of what’s possible.”
Travis Greenhalgh, CEO of SurGenTec [1]
In March 2024, SurGenTec announced the FDA clearance and first implantations of their new OsteoFlo HydroPutty Synthetic Bone Graft. This innovative product offers a fully synthetic bone graft option that is easy to handle, resorbable, and can be used in various surgical procedures. “OsteoFlo HydroPutty represents an advancement in bone graft technology, featuring a combination of proprietary hydrophilic carriers designed to absorb fluids such as bone marrow aspirate, blood or saline. This formulation turns to a putty and offers superior handling characteristics with a resorption profile optimized for bone growth.” By eliminating the risks associated with human tissue, OsteoFlo HydroPutty provides a safer and more reliable solution for bone grafting.[2]
In October 2023, SurGenTec recieved FDA clearance for the TiLink-P™ chronic sacroiliac (SI) joint fusion system, a standalone device for treating chronic sacroiliac joint pain.This innovative fusion system combines effective compression, a minimally invasive approach, and bone grafting to improve healing and patient outcomes. The TiLink-P device consists of a transfixing compression anchor and a locking screw, designed to stabilize the SI joint and promote bone growth. “The locking screw of the TiLink-P system is constructed from 3D-printed titanium. The material offers strength and durability while also incorporating Nanotex surface technology. The hydrophilic surface created by Nanotex, a nanotechnology-based textile enhancements provider, enhances the implant’s osteointegration or the bonding of the implant with the surrounding bone.” Rigorous testing has validated its effectiveness and safety, making it a promising solution for patients with SI joint disorders. Compared to pre-implantation levels, compressive forces were over 500% higher just one hour after implantation.[3] (I was unable to locate raw data / summary reporting to confirm this number.)
References
[1] SurGenTec Wins Two Spine Technology Awards for 2024. (2024, September 18). BusinessWire. https://www.businesswire.com/news/home/20240918487263/en/
[2] FDA clears hydrophilic Synthetic bone graft putty. (2024, March 7). Medical Design & Development. https://www.medicaldesigndevelopment.com/topics/surgical/news/22889442/fda-clears-hydrophilic-synthetic-bone-graft-putty
[3] Medical Device Network. (2024, July 4). SurGenTec’s TILink-P SI Joint Fusion System, US. https://www.medicaldevice-network.com/projects/surgentecs-tilink-p-si-joint-fusion-system/?cf-view
IMAGE — https://www.businesswire.com/news/home/20240918487263/en/
Leave a comment