Disclaimer: This information is for general knowledge only and does not constitute medical advice. Please consult a healthcare professional for any questions or concerns regarding your specific situation.
Medical Device Safety Communications [1]

Summary of FDA Safety Communication Regarding Hintermann Series H3 Total Ankle Replacement (February 29, 2024): [2]
Higher Failure Rate:
- Early data shows a higher percentage of patients requiring additional surgery (up to 28.5%) compared to pre-approval studies (9.9%).
- The FDA and the manufacturer are analyzing various data sources to understand the cause of this increase.
Patient Recommendations:
- Discussions with Doctor:
- Explore alternative treatments for ankle arthritis.
- Understand the risks and benefits of any joint replacement procedure.
- Existing H3 System:
- No removal surgery recommended if the ankle functions well without new problems.
- Seek medical attention for new/worsening pain, swelling, difficulty using the ankle, grinding noises, or weakness around the implant.
- Additional Information:
- Doctors might perform examinations, X-rays, or CT scans to assess the device.
- Report any issues with the H3 system to the FDA to improve patient safety.
Healthcare Provider Recommendations:
- Share Information: Discuss the patient recommendations mentioned above.
- Informed Decision-Making: Involve patients in treatment decisions by explaining all options for ankle arthritis.
- Increased Risk: Be aware of the higher device failure rate observed with the H3 system.
- Follow Instructions: Strictly adhere to the official guidelines for using the H3 system.
- Monitor Patients: Regularly check for potential issues like loosening or fractures in the implanted components.
- Imaging for Suspected Problems: Utilize X-rays and potentially CT scans to assess suspected issues, being mindful that fractures might not be easily detectable.
- Reporting: Report any complications or problems experienced by patients with the H3 system to the FDA.
Additional Information:
- Mobile-Bearing TARs: These ankle replacements consist of metal components and a plastic insert.
- Purpose: Used for replacing painful arthritic ankle joints.
- Hintermann Series H3: Approved in 2019 with ongoing studies.
- FDA Actions: The FDA is working with the manufacturer to investigate the cause of the high failure rate.
Summary of FDA warning regarding the Equinoxe Shoulder System (January 16, 2024). [3]
Key Points:
- Affected Devices: Equinoxe Shoulder Systems manufactured by Exactech between 2004 and August 2021.
- Risk: These implants were packaged in defective bags lacking proper oxygen protection.
- Potential Issue: Exposure to oxygen can degrade the implant material (oxidation) leading to:
- Faster wear and tear
- Cracking or fracture of components
- Possible Consequence: Additional surgery might be needed to replace or fix the device.
Patient Recommendations:
- No surgery for well-functioning implants: If your shoulder feels good and you have no new issues, the FDA advises against removing the device.
- Seek medical attention if you experience:
- New or worsening pain/swelling
- Difficulty using your arm
- Grinding noises near the implant
- Weakness around the implanted area
Healthcare Provider Recommendations:
- Do not implant: Avoid using any Equinoxe Shoulder System with defective packaging.
- Avoid unnecessary removal: Based on current data, the FDA advises against removing well-functioning implants unless new symptoms arise.
- Monitor patients: Regularly assess patients with these implants (2004-2021) for potential problems like wear, failure, or bone loss. X-rays might be used for further evaluation.
- Revision surgery: Discuss the possibility of revision surgery (replacing the device) on a case-by-case basis with patients experiencing worsening pain or weakness potentially linked to the implant.
- Shared decision-making: Involve patients in the decision process by explaining the benefits and risks of all available treatment options.
Additional Information:
- Device Purpose: Replacement of painful shoulder joints due to various conditions.
- Defective Packaging: Certain implants lacked proper oxygen protection, increasing the risk of oxidation.
- Potential Issues due to Oxidation: Increased wear, component fracture, device failure.
- Symptoms: New or worsening pain, swelling, potentially requiring revision surgery.
- Bone Loss: Oxidation might also contribute to bone loss around the implant.
Summary of FDA Warning Regarding Synovo Total Hip System (January 3, 2024) [4]
Issue: Unauthorized modifications were made to the Synovo Total Hip System, raising safety concerns as its effectiveness is not established.
Components Affected:
- Femoral Resurfacing Cup
- Acetabular Fixation Cup
- Acetabular Bearing
Alternative Names:
- Total Hip Replacement System
- Synovo Preserve
- Endotec BP
Recommendations for Patients (implanted after 2019):
- Seek medical attention:
- New or worsening pain
- Loosening of the implant
- Grinding noises
- Difficulty bearing weight
- Weakness in hip/knee on implanted side
- No immediate removal for well-functioning implants: No surgery advised if no new issues arise.
- Continue follow-up appointments: Maintain scheduled checkups with healthcare providers.
Recommendations for Healthcare Providers:
- Do not use: Avoid implanting or purchasing the Synovo Total Hip System.
- Remove existing inventory:
- Femoral Resurfacing Cup
- Acetabular Fixation Cup
- Acetabular Bearing
- Monitor patients with existing implants:
- No removal for well-functioning devices.
- Monitor for potential issues:
- Bone loss
- Loosening
- Wear or failure
- Use X-rays for suspected device failure.
- Communication with patients: Discuss treatment options and risks/benefits of alternatives.
- Review patient recommendations: Inform patients who received the implant about the above recommendations.
Device Description:
- Purpose: Used in total hip replacements.
- Components:
- Femoral Resurfacing Cup
- Acetabular Fixation Cup
- Acetabular Bearing
- Additional Compatibility: Can be used with a standard femoral stem and head component.
FDA Actions:
- Discovery (2022): Identified unauthorized modifications to the system.
- Enforcement Action: Issued a Warning Letter to Synovo demanding:
- Halt to manufacturing modified devices.
- Corrective actions to address the issues.
- Further Steps:
- Requested Synovo to inform customers about the risks.
- Remains engaged with Synovo for compliance.
- Will update the public if significant new information arises.
2024 Medical Device Recalls [5]
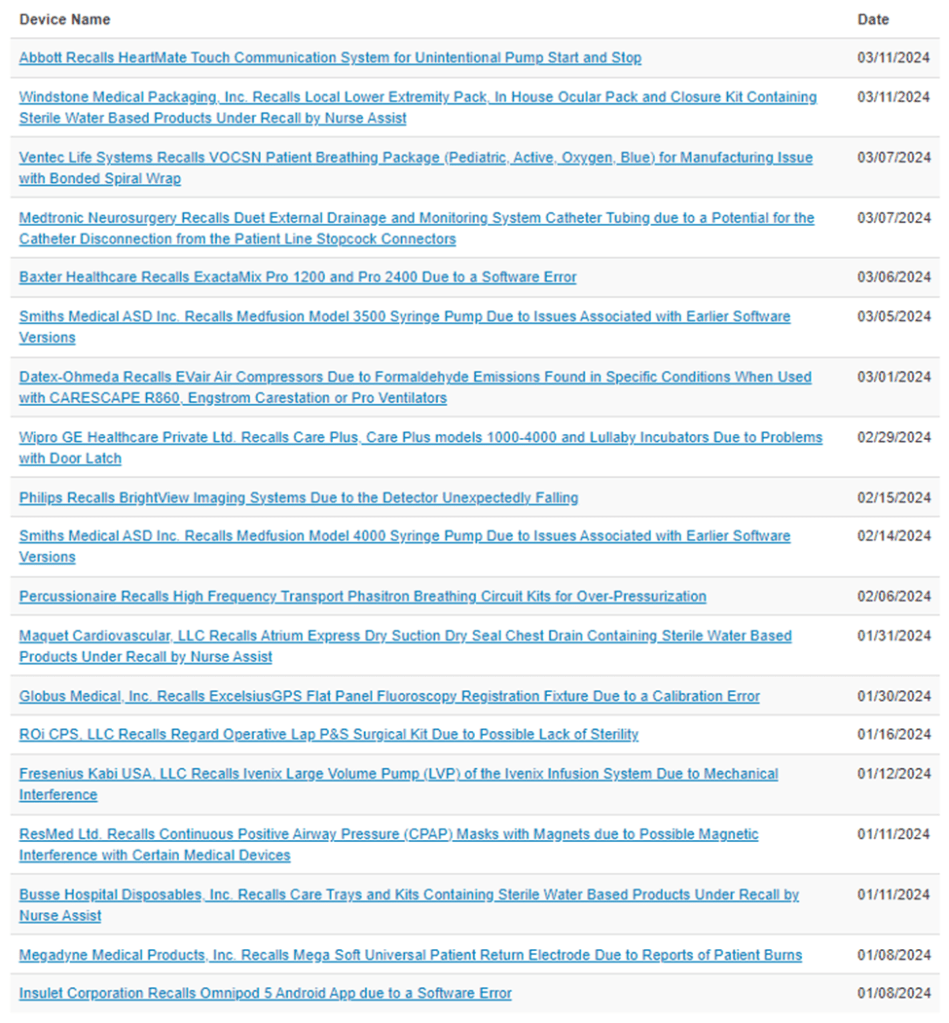
References
[1] Center for Devices and Radiological Health. (2024, February 29). 2024 Safety Communications. U.S. Food And Drug Administration. https://www.fda.gov/medical-devices/safety-communications/2024-safety-communications
[2] Center for Devices and Radiological Health. (2024b, February 29). Hintermann Series H3 total ankle replacement has a Higher-Than-Expected Risk of Device failure: FDA Safety communication. U.S. Food And Drug Administration. https://www.fda.gov/medical-devices/safety-communications/hintermann-series-h3-total-ankle-replacement-has-higher-expected-risk-device-failure-fda-safety
[3] Center for Devices and Radiological Health. (2024a, January 16). Risks with Exactech Equinoxe Shoulder System with Defective Packaging – FDA Safety Communication. U.S. Food And Drug Administration. https://www.fda.gov/medical-devices/safety-communications/risks-exactech-equinoxe-shoulder-system-defective-packaging-fda-safety-communication
IMAGE also taken from this resource
[4] Center for Devices and Radiological Health. (2024a, January 3). Do not use Synovo Total HIP resurfacing System: FDA Safety communication. U.S. Food And Drug Administration. https://www.fda.gov/medical-devices/safety-communications/do-not-use-synovo-total-hip-resurfacing-system-fda-safety-communication
[5] Center for Devices and Radiological Health. (2024e, March 11). 2024 medical device recalls. U.S. Food And Drug Administration. https://www.fda.gov/medical-devices/medical-device-recalls/2024-medical-device-recalls

Leave a comment